Signalment:
7-month-old male neutered Plott Hound
History:
The patient presented to the GCVS Cardiology Service for evaluation of a heart murmur heard at a veterinary visit as a young puppy. No clinical signs at home.
Physical exam:
MM/CRT: pink, moist, <2s
Murmur: Grade V/VI left basilar continuous murmur. Gallop sound noted at LV apex.
Rhythm: Regular rhythm, no extrasystoles noted
HR: 124 bpm
Femoral arterial pulses: Hyperdynamic femoral pulses bilaterally, synchronous
RR/RE: 20, eupneic
Bronchovesicular sounds: clear in all lung fields
Jugular veins: WNL
diagnostics:
- Complete blood count: HCT 43.7 %, WBC 9 k/uL, Plt 222 k/uL; unremarkable
- Serum chemistry: BUN 16 mg/dL, creatinine 0.8 mg/dL; Glc 102 mg/dL; unremarkable
- Pre-operative thoracic radiographs (Figure 1): Marked cardiomegaly with pulmonary vascular overcirculation. A mild diffuse bronchial pattern is noted. No evidence of left-sided congestive heart failure.
- Pre-operative echocardiography (Figure 2):
- The left ventricle is moderately dilated at end-diastole and severely dilated at end-systole. Normal fractional shortening and ejection fraction. Normal diastolic assessment. Normal estimated filling pressures.
- The left atrium is moderately enlarged.
- The right ventricle is normal in size and function.
- The right atrium is normal in size.
- The aortic valve is structurally normal with no subvalvular ridge of tissue and trace AI. The LVOT velocity is mildly elevated (2.2 m/s). This is thought secondary to the increased stroke volume secondary to the left-to-right shunt given the normal valve appearance.
- The mitral valve structure appears normal. There is trace to mild central mitral regurgitation due to annular dilation. Unable to interrogate with spectral Doppler.
- The tricuspid valve appears normal. There is trace tricuspid regurgitation.
- The pulmonic valve is normal. The RVOT velocity is normal. The PA is dilated due to the continuous high velocity L-R flow.
- There is no pericardial effusion.
- There is a moderately sized L-to-R shunting patent ductus arteriosus (PDA) present. Minimal ductal diameter measures around 3.5-4.2 mm. There is a dilated ampulla with relatively acute tapering at the pulmonary insertion site, consistent with a Miller type 2 ductal morphology (A vs. B unable to classify on TTE). Normal transductal velocity (5.1 m/s) without evidence of significantly elevated PA pressures. No other congenital defects present.
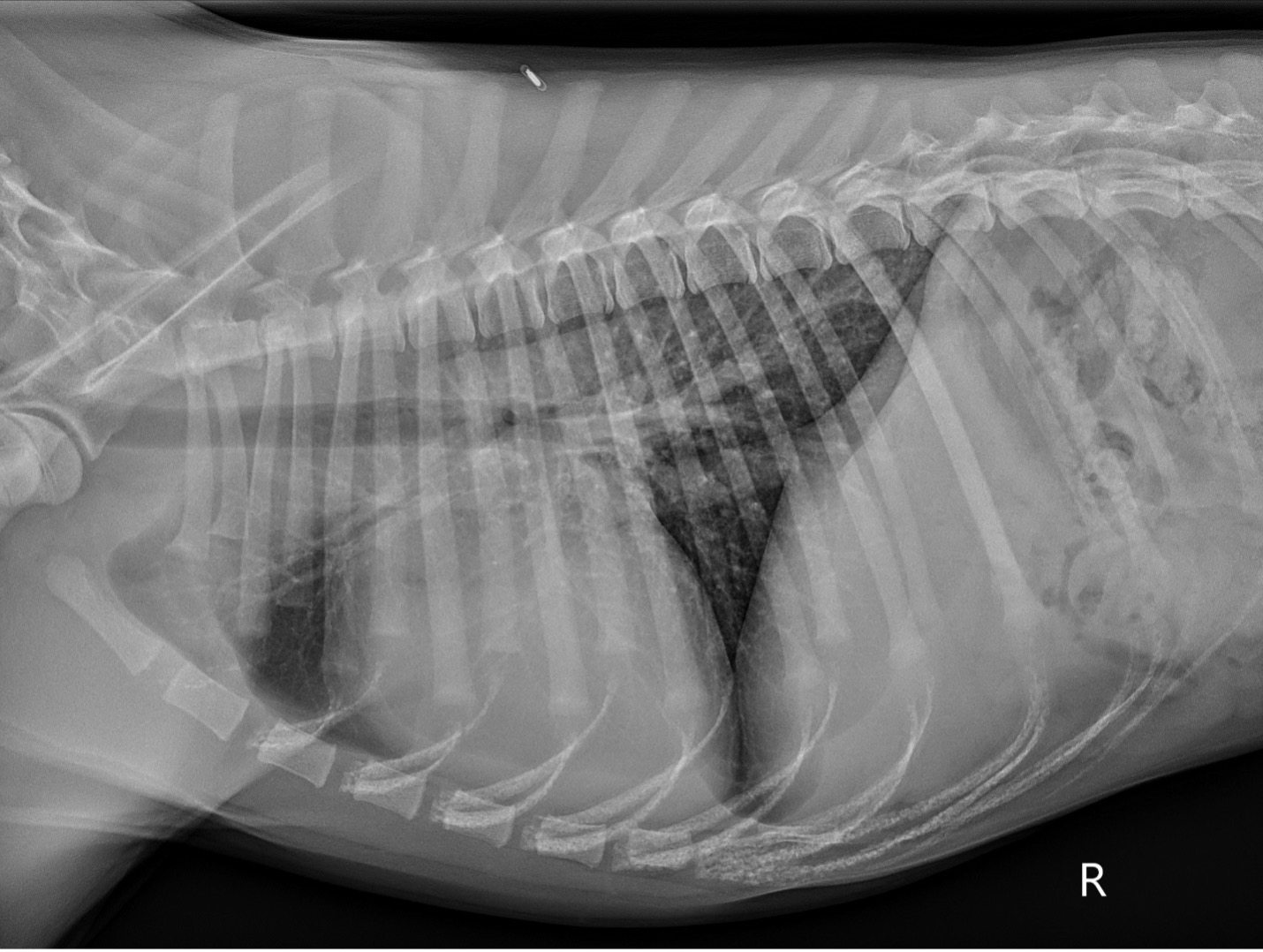 PICTURE:
PICTURE: Figure 1: Pre-operative right lateral thoracic radiograph.
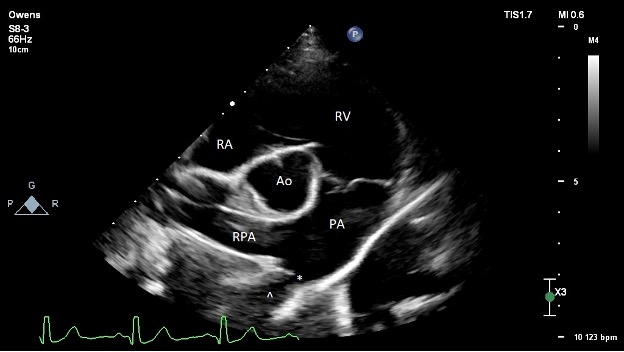 PICTURE:
PICTURE: Figure 2: Pre-operative transthoracic echocardiography image obtained from the right parasternal short axis at the level of the heart base. The ostium of the PDA is denoted with an asterisk (*). The ampulla of the ductus is denoted with a caret (^). RA = right atrium; RV = right ventricle; PA = main pulmonary artery; RPA = right pulmonary artery; Ao = aorta
Procedure
The patient was anesthetized and placed in right lateral recumbency. The right medial thigh was clipped, prepped, and surgically draped. A 3 cm skin incision was performed centering over the right proximal femoral artery using a #10 blade. The subcutaneous tissue superficial to the right femoral artery was dissected with hemostats and Metzenbaum scissors. An approximately 2 cm section of the right femoral artery was isolated by placing vascular loops both proximally and distally. The distal femoral artery was ligated with a single encircling suture using 3-0 Nylon. An 18 gauge over-the-needle IV catheter was used to gain access to the femoral artery. A 0.035” x 145 cm guidewire was advanced through the catheter into the right femoral artery, and the catheter was removed. A 7 Fr x 90 cm introducer sheath was advanced over the guidewire into the lumen of the right femoral artery and into the thoracic aorta with fluoroscopic guidance. The guidewire and dilator we removed. A 5 Fr x 100 cm MPA catheter was advanced through the sheath and into the proximal descending aorta. A hand injection of 10 mL of iodinated contrast was performed through the MPA, which highlighted the PDA well and the pulmonary ostium was measured (3.5 mm – 4.2 mm).
The 5 Fr MPA catheter was used to guide the sheath across the ductus and into the main pulmonary artery. The MPA catheter was subsequently removed. A 7 mm Amplatz Canine Ductal Occluder (ACDO) was advanced into the delivery sheath. The distal disc of the ACDO was extruded from the catheter while within the main pulmonary artery. Both the introducer and device were retracted until gentle tension was felt. The remainder of the device was deployed within the ductal ampulla. Gentle pushing and pulling on the cable was performed to confirm placement and stability of the device. The device was allowed to remain stationary for approximately 5 minutes while its location was confirmed with TEE. No residual flow was seen after 5 minutes. A hand injection of 10 mL of iodinated contrast was performed through the introducer sheath, and no residual ductal flow was noted angiographically. The device was then released from the cable, and the cable was removed. The delivery sheath was removed. Two encircling sutures were placed around the proximal femoral artery with 3-0 Nylon. The previously placed vascular loops were removed. The exposed muscle and fascia were apposed with 3-0 Monocryl in a simple continuous pattern. The subcutaneous tissue was apposed with 3-0 Monocryl in a simple continuous pattern. The skin was apposed using 3-0 Nylon.
Video: Ferb PDA post angio.
Post-operative thoracic radiographs (Figure 3): Improved cardiomegaly with reduction in pulmonary vascular overcirculation. Similar mild diffuse bronchial pattern. ACDO device in native shape in the ductus arteriosus.
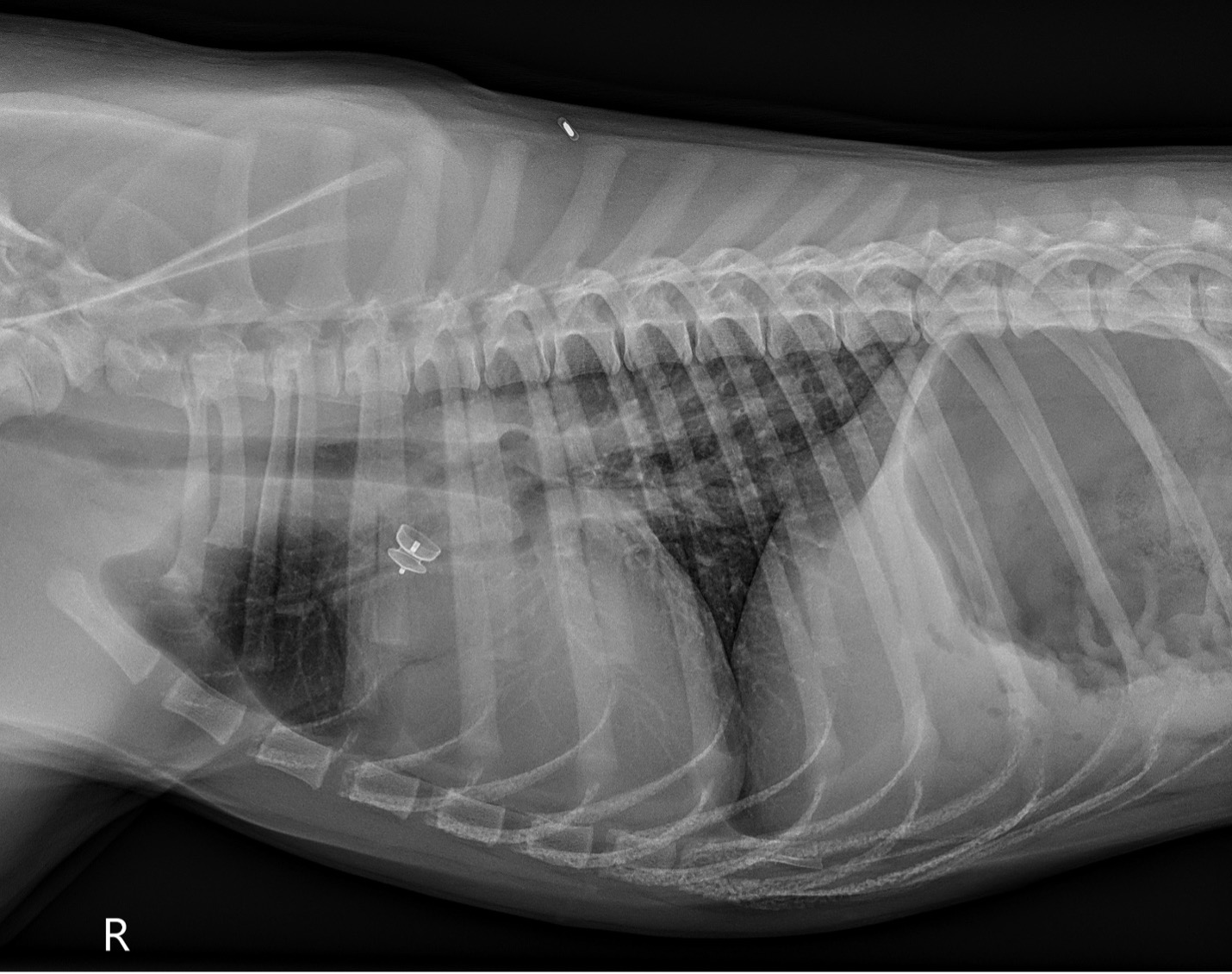 PICTURE:
PICTURE: Figure 3: Post-operative right lateral thoracic radiograph.
Post-procedure echocardiography (Figure 4):
- The left ventricle is normal at end-diastole, and high-normal at end-systole. Low-normal fractional shortening and ejection fraction. Normal diastolic assessment.
- The left atrium is normal in size.
- The right ventricle is normal in size and function.
- The right atrium is normal in size.
- The aortic valve is structurally normal. No AI noted. The previously observed elevated LVOT velocity is resolved, confirming the pre-operative suspicion of volume related increased flow velocity.
- The mitral valve structure appears normal. There is trivial central mitral regurgitation. Unable to interrogate with spectral Doppler.
- The tricuspid valve appears normal. There is trace tricuspid regurgitation.
- The pulmonic valve is normal. The RVOT velocity is normal. The PA is subjectively mildly dilated. Mild PI, PI Vmax 1.6 m/s suggests normal estimated mean PA pressures.
- There is no pericardial effusion.
- The is an ACDO device in place within the ductus arteriosus that appears to maintain its native shape. There is no residual ductal flow observed.
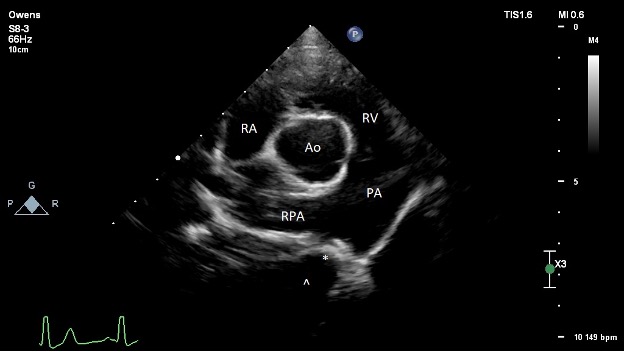 PICTURE:
PICTURE: Figure 4: Post-operative transthoracic echocardiography image obtained from the right parasternal short axis at the level of the heart base. The ACDO device is observed in the PDA, denoted with an asterisk (*). The ampulla of the ductus is denoted with a caret (^). RA = right atrium; RV = right ventricle; PA = main pulmonary artery; RPA = right pulmonary artery; Ao = aorta.
Outcome:
Successful occlusion of the PDA. Normal cardiac size and function on follow-up echocardiography performed with another cardiologist. The patient is expected to have a normal life expectancy.
Patent ductus arteriosus
This condition is usually inherited as a genetic trait and female puppies and certain breeds (Collie, Shetland sheepdog, German shepherd, Maltese, Yorkshire terrier, and Labrador) appear more frequently affected. This defect is due to the persistence of the ductus arteriosus, a vascular structure that is important during fetal development. The ductus arteriosus is a small vessel that connects the pulmonary artery and the aorta. In the womb, the ductus arteriosus is responsible for delivering oxygenated blood into systemic circulation, bypassing the non-functional lungs.
In the normal animal, the ductus arteriosus closes fully within the first 7 to 10 days of life. In some animals, the ductus arteriosus does not close, remaining patent. This is likely due to abnormalities in vascular smooth muscle in this tissue. When this happens, blood from the aorta flows into the pulmonary artery, causing a left to right shunt and left-sided volume overload. Most animals with a moderate or large left to right shunting PDA develop signs of congestive heart failure due to overload of the pulmonary circulation and the left side of the heart. Without treatment approximately 2/3 of dogs with a PDA will die before reaching one year of age from congestive heart failure.
When detected and treated early with closure, most dogs can go on to live a normal life. There are two ways to achieve closure of a PDA. Historically, surgery has been performed to manually ligate the PDA. This approach involves a thoracotomy and gentle dissection to expose the vessel and place suture material around the outside of the vessel. This approach is often very successful, but there is a risk for severe bleeding complications as well more discomfort associated with the large incision. A more recent advancement in veterinary cardiology is the minimally invasive placement of a special occlusion device (Amplatz Canine Ductal Occluder (ACDO)) within the ductus arteriosus. This procedure is performed with a veterinary cardiologist and involves a very small incision over the femoral artery to allow placement of a delivery catheter across the ductus arteriosus. The use of fluoroscopy and transesophageal echocardiography help with visualization and deployment of the device. This procedure is extremely successful with a decreased risk of catastrophic complications at the time of surgery when compared with surgical ligation. The weight of the patient, size of the ductus arteriosus, as well as the anatomy of the ductus arteriosus sometimes factor into the recommendation for surgery versus a minimally invasive occlusion procedure.
 PICTURE: Figure 1: Pre-operative right lateral thoracic radiograph.
PICTURE: Figure 1: Pre-operative right lateral thoracic radiograph.
 PICTURE: Figure 2: Pre-operative transthoracic echocardiography image obtained from the right parasternal short axis at the level of the heart base. The ostium of the PDA is denoted with an asterisk (*). The ampulla of the ductus is denoted with a caret (^). RA = right atrium; RV = right ventricle; PA = main pulmonary artery; RPA = right pulmonary artery; Ao = aorta
PICTURE: Figure 2: Pre-operative transthoracic echocardiography image obtained from the right parasternal short axis at the level of the heart base. The ostium of the PDA is denoted with an asterisk (*). The ampulla of the ductus is denoted with a caret (^). RA = right atrium; RV = right ventricle; PA = main pulmonary artery; RPA = right pulmonary artery; Ao = aorta
 PICTURE: Figure 3: Post-operative right lateral thoracic radiograph.
PICTURE: Figure 3: Post-operative right lateral thoracic radiograph.
 PICTURE: Figure 4: Post-operative transthoracic echocardiography image obtained from the right parasternal short axis at the level of the heart base. The ACDO device is observed in the PDA, denoted with an asterisk (*). The ampulla of the ductus is denoted with a caret (^). RA = right atrium; RV = right ventricle; PA = main pulmonary artery; RPA = right pulmonary artery; Ao = aorta.
PICTURE: Figure 4: Post-operative transthoracic echocardiography image obtained from the right parasternal short axis at the level of the heart base. The ACDO device is observed in the PDA, denoted with an asterisk (*). The ampulla of the ductus is denoted with a caret (^). RA = right atrium; RV = right ventricle; PA = main pulmonary artery; RPA = right pulmonary artery; Ao = aorta.